39 phase labels in chemical equations
study.com › academy › lessonHow to Balance Chemical Equations - Study.com Jan 16, 2022 · write a balanced chemical equation including phase labels for the reaction between aqueous copper (II) nitrate and aqueous sodium hydroxide. Write a balanced overall reaction from these unbalanced ... Solved Part 7: Aqueous Chemistry...Ionic Equations 7. In | Chegg.com In many aqueous chemical reactions, there are ions that are not involved in the chemical change but serve to deliver the ions that are involved. hen a compound in a chemical equation has an aqueous label, and is ionic, it consists of the constituent ions separated in solution. We represent the ions in a complete This question hasn't been solved yet
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways

Phase labels in chemical equations
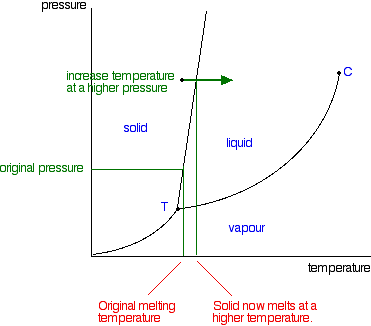
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution Factor-Label Method in Chemistry: Steps and Conversions - Study.com Multiply by the numbers in the numerator and divide by those in the denominator. {eq}\frac { (3) (1)} {2.2}=1.4 {/eq} Since the units left in the problem are kilograms the answer is 1.4 kg. This... State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
Phase labels in chemical equations. GCSE CHEMISTRY - What are State Symbols? - (s) - (l) - (g) - (aq ... The complete equations in words and symbols would be potassium + chlorine potassium chloride. 2 K (s) + Cl 2(g) 2 K Cl (s) lith ium + oxygen lithium oxide. 4 Li (s) + O 2 (g) 2 Li 2 O (s) The state symbols in brackets show the physical state of the substance at the reaction temperature. Solid (s), liquid (l), gas (g), or dissolved in water (aq). › questions-and-answers › completeAnswered: Complete and balance the following… | bartleby Solution for Complete and balance the following molecular equations (indicate the relevant phase labels): LICI (s) + HSO4 (aq) → NH. (aq) + OH(aq) → AgNOs (aq)… Symbols in Chemical Equations - Harper College Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read en.wikipedia.org › wiki › Droplet-based_MicrofluidicsDroplet-based microfluidics - Wikipedia Surfactants play an important role in droplet-based microfluidics. The main purpose of using a surfactant is to reduce the interfacial tension between the dispersed phase (droplet phase, typically aqueous) and continuous phase (carrier liquid, typically oil) by adsorbing at interfaces and preventing droplets from coalescing with each other, therefore stabilizing the droplets in a stable ...
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ) 4.1 Writing and Balancing Chemical Equations | Chemistry - Lumen Learning 4 × 1 = 4. 2 × 2 = 4. 4 = 4, yes. O. 2 × 2 = 4. (1 × 2) + (2 × 1) = 4. 4 = 4, yes. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. Answer N 2 3H 2 2NH 3 Many chemical equations also include phase labels ... Answer N 2 3H 2 2NH 3 Many chemical equations also include phase labels for the. Answer n 2 3h 2 2nh 3 many chemical equations also. School ETI Technical College; Course Title NURS 302; Uploaded By Tgayle08. Pages 855 Ratings 100% (1) 1 out of 1 people found this document helpful; Chemical Reactions and Equations - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.
How do you Write a Chemical Equation? - A Plus Topper At the microscopic level, the coefficients in a chemical reaction tell us the number of particles 1 involved in the reaction. A chemical equation serves as an important ; communicative tool for chemists. (a) A chemical equation precisely describes a chemical reaction. (b) Chemists use chemical equations to solve quantitatively-related problems ... pubs.acs.org › toc › jceda8/99/2Journal of Chemical Education | Vol 99, No 2 - ACS Publications Feb 08, 2022 · Electroreduction of CO2 to useful chemicals is a rapidly developing field of study and one of the foundational technologies for a carbon-neutral future. Integrating laboratory practice on this topic in undergraduate chemistry curricula is an excellent opportunity to improve student exposure and basic training in synthetic electrochemistry. In the article, the authors describe a laboratory ... Phase diagram - Wikipedia The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas. The curves on the phase diagram show the points where the free energy (and other derived properties) becomes non-analytic: their derivatives with respect to the coordinates (temperature and pressure in this example) change discontinuously (abruptly). Definition of dissolve and the state label (aq) in chemical equations C O X 2 ( a q) would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). B r X 2 ( a q) would imply homogeneous solution of liquid bromine in water. N a C l ( a q): Salt dissolved in water. A colloid is a heterogeneous phase.
diffeq.sciml.ai › stable › tutorialsOrdinary Differential Equations · DifferentialEquations.jl AutoVern7(Rodas5()) handles both stiff and non-stiff equations in a way that's efficient for high accuracy. Tsit5() for standard non-stiff. This is the first algorithm to try in most cases. BS3() for fast low accuracy non-stiff. Vern7() for high accuracy non-stiff. Rodas4() or Rodas5() for small stiff equations with Julia-defined types, events ...
Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note
en.wikipedia.org › wiki › Scientific_lawScientific law - Wikipedia Scientific laws or laws of science are statements, based on repeated experiments or observations, that describe or predict a range of natural phenomena. The term law has diverse usage in many cases (approximate, accurate, broad, or narrow) across all fields of natural science (physics, chemistry, astronomy, geoscience, biology).
3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number and type of atoms on the left side of the arrow is the same as the number of type of atoms on the ...
Phase Rule - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases.
Phase plane - Wikipedia Phase plane. In applied mathematics, in particular the context of nonlinear system analysis, a phase plane is a visual display of certain characteristics of certain kinds of differential equations; a coordinate plane with axes being the values of the two state variables, say ( x, y ), or ( q, p) etc. (any pair of variables).
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.
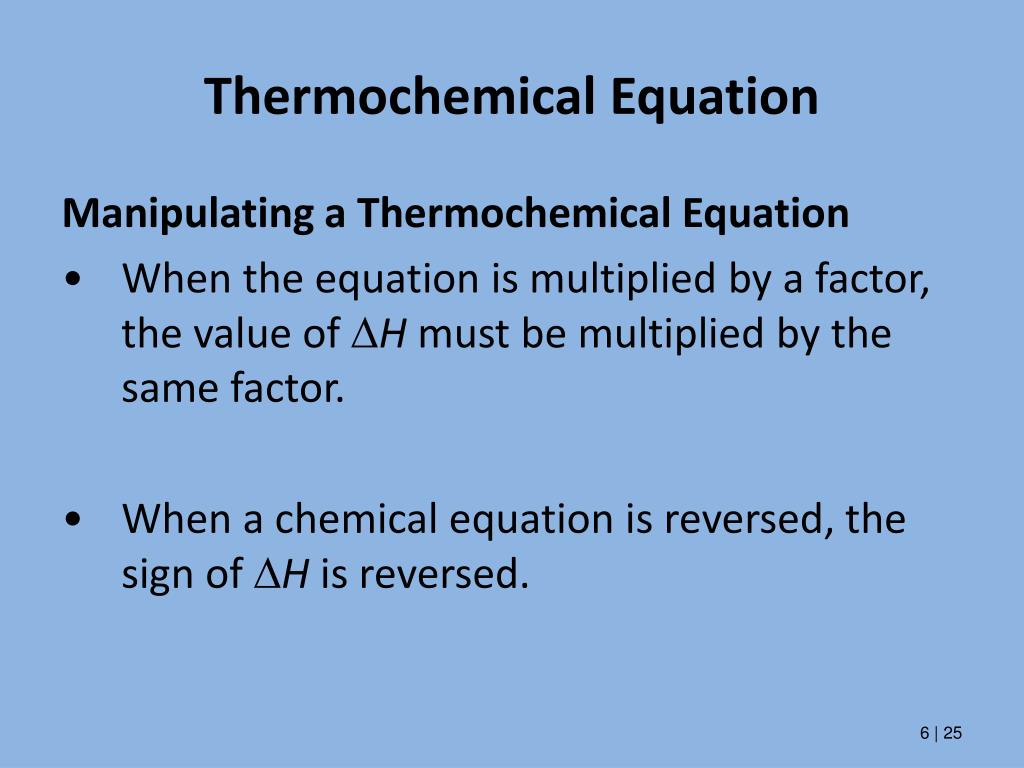
A thermochemical equation is a balanced chemical reaction equation ... A thermochemical equation is a balanced chemical reaction equation (including phase labels) with the enthalpy of reaction value written directly after the equation THERMOCHEMICAL EQUATIONS A reaction that produces heat is referred to as exothermic. For exothermic reactions, enthalpy values are assigned a negative value (-DH).
What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ...
› chemistry-solversChemistry Problem Solver Online | Chemistry Homework Help ... If those strange equations a chemistry teacher writes on a chalkboard drive you crazy, there’s no need in torturing yourself. Thanks to the digital and technological progress, you can find a chemistry problem solver for any occasion. Here are some you can use. Chem Wiz; pH Calculator; Balancing Chemical Equations; Mole Calculator
Chapter 7 Now we'll use the Solubility Table to predict the phases of the products. According to the table CaSO 4 is INSOLUBLE and NaCl is SOLUBLE. Na 2 SO 4(aq) + CaCl 2(aq)---> CaSO 4(s) + 2NaCl (aq) Let's consider another example. Write the formula and identify the phase for the product(s) and balance the following reaction. AgNO 3(aq) + Na 2 CO 3(aq)--->
Using state symbols in chemical equations - BBC Bitesize Learn about and revise equations and chemical reactions with this BBC Bitesize GCSE Combined Science (OCR 21C) study guide.
Many chemical equations also include phase labels for Many chemical equations also include phase labels for. School Eastern Visayas State University - Tacloban City Main Campus; Course Title AGRICULTUR 847,272; Uploaded By MasterPencil4687. Pages 46 This preview shows page 25 - 27 out of 46 pages. ...
End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Phase Definition and Examples - ThoughtCo In chemistry and physics, a phase is a physically distinctive form of matter, such as a solid, liquid, gas, or plasma. A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter.
State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)


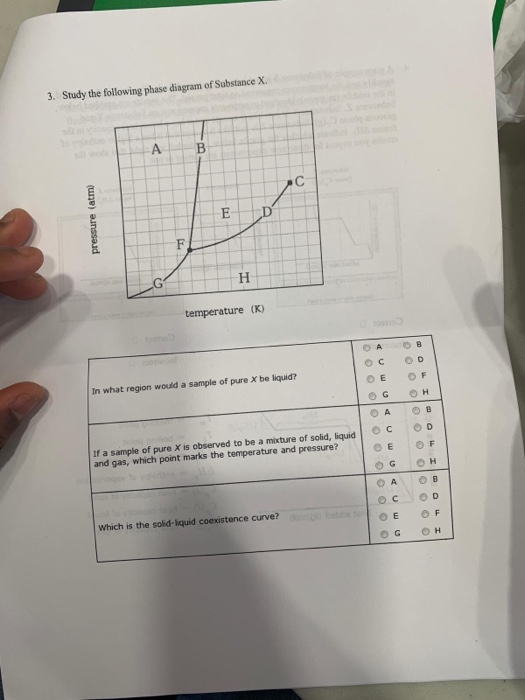
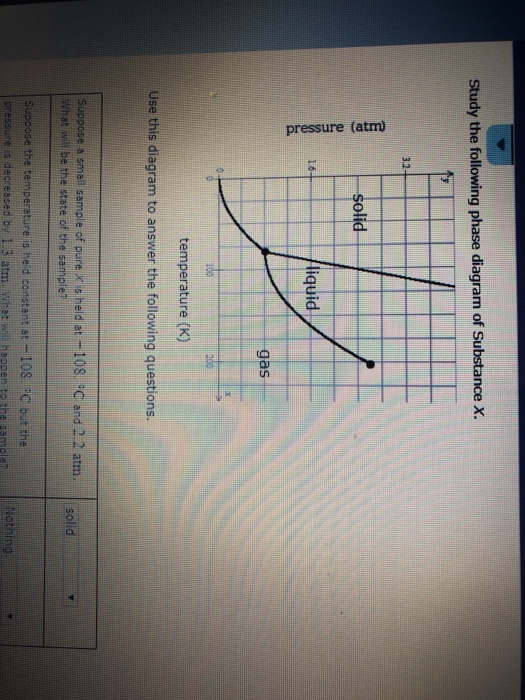


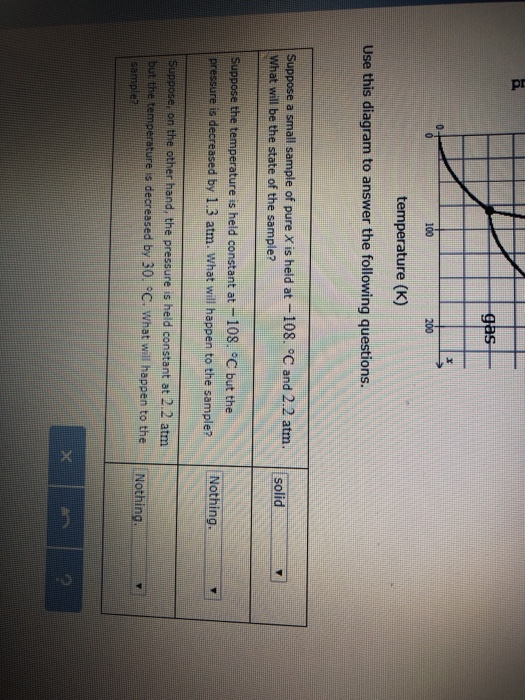



Post a Comment for "39 phase labels in chemical equations"